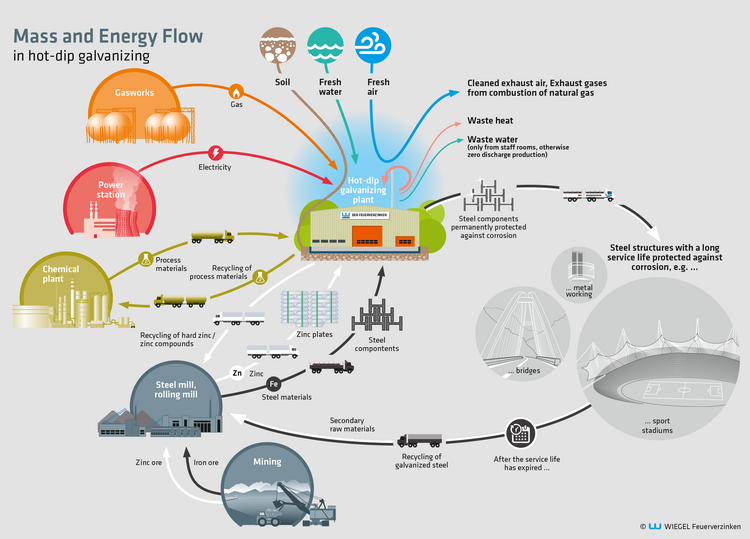
Hot-dip galvanization is a “hot dipping process”,
Hot-dip galvanization is a “hot dipping process”, which involves metallic workpieces (almost always steel) are dipped into a vat of molten zinc at a temperature of 450 °C for several minutes. This causes iron-zinc alloys of varying composition to form on the wetted surfaces of the workpiece, which protect the workpiece against corrosion (rust) for decades. This process causes emissions of exhaust gases, smoke and dust.
To keep a volume
To keep a volume – depending on the size of the plant – of approx. 40 to 100 m3 of zinc in a zinc kettle in a molten state permanently, a considerable amount of heat energy is required, some of which escapes into the atmosphere as waste heat.
Prior to the actual galvanization,
Prior to the actual galvanization, a chemical pre-treatment of the workpiece is also required, which is also a dipping procedure performed in large baths and which we will go into further detail later. This also results in fumes (hydrochloric acid, ammonia).